You are here: Home: NHLU 5 2005 : Mark S Kaminski, MD
| CD 1 — Tracks 17-22; CD 2 — Tracks 1-4 |
| Track 17 |
Radioimmunotherapy as first-line
treatment of follicular lymphoma |
| Track 18 |
Bcl-2 translocation as a predictor
of PCR negativity and response
to radioimmunotherapy |
| Track 19 |
Interpreting radioimmunotherapy
data in comparison to other clinical
trials of first-line therapy |
| Track 20 |
Acute and long-term toxicities
associated with radioimmunotherapy |
| Track 21 |
SWOG trial of CHOP followed
by Bexxar® consolidation in
responding patients |
|
| Track 22 |
SWOG trial S0016 comparing
R-CHOP versus CHOP followed
by Bexxar in patients with newly
diagnosed follicular NHL |
| CD 2 |
|
| Track 1 |
Potential strategies for evaluating
the optimal use of radioimmunotherapy |
| Track 2 |
Treatment algorithm for newly
diagnosed indolent lymphoma |
| Track 3 |
Incorporation of radioimmunotherapy
in the treatment of
indolent lymphoma |
| Track 4 |
Evaluating immunologic and novel>
targeted therapies in NHL |
|
|
Select Excerpts from the Interview*
 CD 1, Track 17 CD 1, Track 17
 DR LOVE: One of the most discussed publications in lymphoma this year
was your paper in The New England Journal of Medicine evaluating radioimmunotherapy
as first-line treatment for follicular lymphoma (Kaminski
2005). Can you comment on the background to that trial? DR LOVE: One of the most discussed publications in lymphoma this year
was your paper in The New England Journal of Medicine evaluating radioimmunotherapy
as first-line treatment for follicular lymphoma (Kaminski
2005). Can you comment on the background to that trial? |
 DR KAMINSKI: We performed a lot of the developmental work on radioimmunotherapy
with CD20 radio-labeled antibodies. We had shown how to
administer it, what its toxicities were and where its greatest value appeared to
be, which was in the follicular lymphomas. We had also done a lot of work in
patients with refractory disease, and it was working in them. DR KAMINSKI: We performed a lot of the developmental work on radioimmunotherapy
with CD20 radio-labeled antibodies. We had shown how to
administer it, what its toxicities were and where its greatest value appeared to
be, which was in the follicular lymphomas. We had also done a lot of work in
patients with refractory disease, and it was working in them.
The natural idea here is that if it works in the back-line setting, it should work
even better in the front-line setting, where there’s less potential resistance
— the patients are more immunocompetent. So especially in an incurable
disease such as follicular lymphoma, it made all the sense in the world to use
something that appeared in some of our trials to be superior to chemotherapy
as front-line therapy.
The trial evaluated Bexxar in patients with Stage III and IV follicular
lymphoma — advanced-stage disease — with no prior treatment. Our first
patient was accrued in June of 1996, so we have quite a long follow-up on
these patients. The first-patient phenomenon worked in this trial: That patient
is still in complete remission.
 DR LOVE: Can you summarize the data that you recently reported in The
New England Journal of Medicine? DR LOVE: Can you summarize the data that you recently reported in The
New England Journal of Medicine?
 DR KAMINSKI: We showed in 76 patients that we could achieve a 95 percent
response rate with only one course of treatment, which only takes a week to
give, and a 75 percent complete remission rate (2.1). We now have long-term
follow-up — the median follow-up is over five years — and the progression-free
survival at five years is 60 percent for all the patients entered on the trial. DR KAMINSKI: We showed in 76 patients that we could achieve a 95 percent
response rate with only one course of treatment, which only takes a week to
give, and a 75 percent complete remission rate (2.1). We now have long-term
follow-up — the median follow-up is over five years — and the progression-free
survival at five years is 60 percent for all the patients entered on the trial.
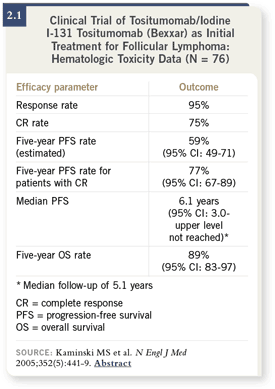 Of those who had a complete
response, more than 70
percent — 75 percent —
were still in remission at
five years. At this point, an
abundant number of patients
are beyond five years. We
only had four relapses, and
of those four, three relapsed
only in a solitary site, and
we treated that with conventional
radiation therapy and
put them back into remission.
That’s one aspect.
Of those who had a complete
response, more than 70
percent — 75 percent —
were still in remission at
five years. At this point, an
abundant number of patients
are beyond five years. We
only had four relapses, and
of those four, three relapsed
only in a solitary site, and
we treated that with conventional
radiation therapy and
put them back into remission.
That’s one aspect.
The other aspect of this trial
was that we were interested
to see if we could induce a
molecular remission. If the
treatment had any chance of
being a potential cure, to be
molecularly negative would
certainly be going a long
way in that direction, so we
actually measured the Bcl-2
translocation using PCR
techniques, serially, in the bone marrow of these patients. We found that of the patients who had the
Bcl-2 translocation, more than 90 percent showed molecularly negative results
at some point in the follow-up.
 CD 1, Track 20 CD 1, Track 20
 DR LOVE: What did you see in terms of short- and long-term toxicities? DR LOVE: What did you see in terms of short- and long-term toxicities? |
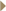 DR KAMINSKI: Myelodysplastic syndrome and acute myelogenous leukemia
are the long-term toxicities we’re most concerned about, and we didn’t see
any — zero — with a median follow-up of five years. DR KAMINSKI: Myelodysplastic syndrome and acute myelogenous leukemia
are the long-term toxicities we’re most concerned about, and we didn’t see
any — zero — with a median follow-up of five years.
As for the short term, the major toxicity is hematological (2.2). It occurs at
about six to seven weeks in terms of the nadir of blood counts, but none of
these patients had to have any transfusions. No one had febrile neutropenia
requiring hospitalization or antibiotics, and none of them received growth
factors; it was very well tolerated.
 CD 2, Track 3 CD 2, Track 3
 DR LOVE: In the clinical management of indolent lymphomas, where
does radioimmunotherapy generally fit into your algorithm? DR LOVE: In the clinical management of indolent lymphomas, where
does radioimmunotherapy generally fit into your algorithm? |
 DR KAMINSKI: I try to utilize it as early as possible in the course of the
disease. If a patient has had a long remission with chemotherapy and then they
require more therapy, we have the options to give just rituximab or go on to
give additional chemotherapy or to give radioimmunotherapy. I would clearly
use radioimmunotherapy in patients who have short responses to chemotherapy
and who have relatively poor responses to rituximab. DR KAMINSKI: I try to utilize it as early as possible in the course of the
disease. If a patient has had a long remission with chemotherapy and then they
require more therapy, we have the options to give just rituximab or go on to
give additional chemotherapy or to give radioimmunotherapy. I would clearly
use radioimmunotherapy in patients who have short responses to chemotherapy
and who have relatively poor responses to rituximab.
In general, because of the simplicity of the treatment and the brevity of it, I
really have a hard time not thinking of radioimmunotherapy for a patient
who has relapsed with a follicular lymphoma if the disease is progressing
and is potentially beginning to become or is symptomatic. That’s where the
highest complete response rates are, and that’s where the duration of response
is greatest. If you obtain a complete response, you have an excellent chance of
remaining that way for five years, and very few chemotherapeutic approaches
out there demonstrate that.
Select publications
* Conducted on April 8, 2005
|

