You are here: Home: NHLU 1 2005 : Fernando Cabanillas, MD
| Fernando Cabanillas, MD |
EDITED COMMENTS |
 Phase III trial of rituximab/FND in patients with Stage IV indolent lymphoma Phase III trial of rituximab/FND in patients with Stage IV indolent lymphoma
Our institution accrued the largest number of patients to the initial multi-institutional, singleagent pivotal trial of rituximab (McLaughlin 1998). We then conducted a trial combining rituximab with FND, and that proved to be the most active combination we’ve ever tried in the front-line management of Stage IV indolent lymphoma (McLaughlin 2000).
Not only did we see a higher clinical complete response rate, but the molecular response rate, as measured by the PCR test for Bcl-2 rearrangement, was also high.
Both arms of the trial included rituximab and FND, but in the first arm the agents were administered simultaneously, whereas the second arm began with eight courses of FND followed by rituximab one year later.
The molecular response rate was 90 percent in the patients who received the agents simultaneously, versus 70 percent in the delayed rituximab arm — and that was statistically significant. Essentially, the molecular responses were good in both arms, but it appears that administering rituximab simultaneously is superior in terms of molecular response.
An important outcome of this study was that, for the first time, we were able to show that molecular remissions are attainable with rituximab combined with chemotherapy in the vast majority of cases. With CHOP alone, few patients achieved a molecular remission — only 15 percent at best. Although we didn’t conduct a randomized study against CHOP, we believe the rituximab/FND regimen is superior based on the high molecular response rate.
Dr Zinzani conducted a trial in Italy comparing CHOP to a combination of fludarabine and mitoxantrone and demonstrated a higher molecular response rate with the fludarabine/mitoxantrone combination. I believe it’s becoming clear that fludarabine in combination is superior to CHOP, at least in terms of the quality of the responses.
Role of the immune system in the prognosis of patients with indolent lymphomas
Investigators are beginning to focus more on benign or normal cells, such as macrophages and lymphocytes, rather than malignant cells. Studies now show that the gene profile of normal cells in patients who do well is different from that of patients who do poorly, and the immune system appears to play an important role (Annuska 2004).
Also, data derived from children with acute lymphoblastic leukemia show that patients who have been in remission for years and are considered cured still have residual tumor cells that are detectable by techniques such as PCR. It appears that, at least in the lymphoid disorders, patients can actually live with a small amount of residual lymphoma or lymphoid leukemia cells, which the immune system is able to keep in check.
Immunologic mechanisms of action of rituximab
We all believe rituximab works through the immune system, but multiple mechanisms may exist. The one that most people agree with is the antibodydependent cell-mediated cytotoxicity (ADCC) mechanism, which depends a lot on the immune system. In this case, rituximab appears to attach to the tumor cell through the Fab fragment of the antibody molecule. The Fc portion then sticks out and is recognized by the immune effector cells that have Fc receptors. These cells are then eliminated from the system.
Other mechanisms may include direct lysis of the tumor cells. Some people even believe that the CD-20 molecule might play an important biological role in keeping the cell alive, and that interfering with this molecule might also lead to cell death, although that’s not been proven.
Development of rituximab in the treatment of NHL
At the time of the pivotal trial of rituximab, it was the first antibody ever used in oncology. We had no experience with any like it, and I was surprised not only that it worked but also that it worked in the majority of patients with follicular lymphoma. We saw approximately a 60 percent response rate with rituximab in the salvage setting, and the rate was even higher in the front-line setting.
In addition, rituximab has a completely different mechanism of action than chemotherapy, and generally we can combine it with chemotherapy without having to compromise the dose of chemotherapy. That’s something we can’t do with most chemotherapy agents because the majority of them are myelotoxic.
It’s been interesting to follow the development of rituximab. While we initially thought it was mostly active in follicular lymphoma, we then learned that other nonfollicular indolent lymphomas are also responsive. For example, marginal zone lymphomas respond to rituximab, and it has been shown that chronic lymphocytic leukemia is another important target for rituximab combined with chemotherapy.
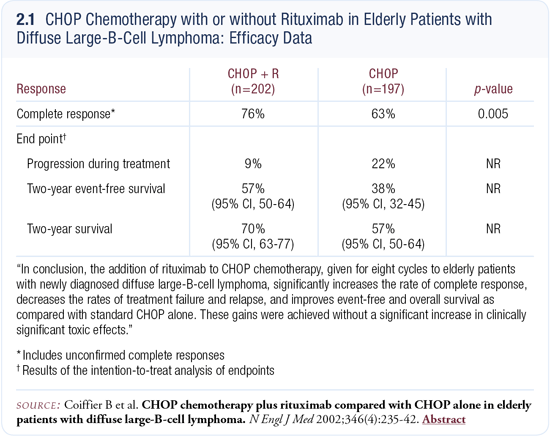
My biggest surprise was with large-cell lymphoma. The single-agent data with large cell lymphoma was not promising, yet when rituximab was combined with CHOP in the front-line setting — as was done by Coiffier in France — rituximab contributed very significantly to the outcome (Coiffier 2002; [2.1]).
Nonprotocol use of maintenance rituximab in indolent lymphoma
In the past, interferon was my drug of choice for maintenance therapy because data showed that, when combined with chemotherapy regimens like CHOP-Bleo, it prolonged failure-free survival (McLaughlin 1993); however, interferon is toxic and not easy for patients to tolerate for one or two years, so I was interested in the idea of maintenance rituximab.
Data demonstrate that rituximab definitely prolongs failure-free survival, but we don’t know whether it prolongs overall survival. Many things can occur after patients relapse on (or after completion of) rituximab that can effect survival.
In addition, the median survival with standard therapy is seven years, and the disease is so indolent it takes a long time to demonstrate an impact on survival.
Although we don’t know whether maintenance rituximab prolongs survival, increasing the duration of failure-free survival is an achievement from a quality-of-life standpoint. I believe an improvement in failure-free survival usually translates into an improvement in overall survival, so I’m not waiting to see if anyone proves that point — I’m already using maintenance rituximab instead of interferon. I administer four weekly doses of rituximab every six months for two years.
Rituximab/FND plus oxaliplatin in patients with relapsed follicular lymphoma
We just began a trial for patients with relapsed follicular lymphoma using a combination of rituximab, fludarabine, oxaliplatin, mitoxantrone and dexamethasone. Basically, it’s an extension of the rituximab/FND regimen that we’ve utilized as salvage therapy and, more recently, in the front-line setting. We added oxaliplatin to try to exploit synergism with fludarabine.
The schedule of administration is similar to the FND/rituximab regimen: fludarabine daily for three days, mitoxantrone and oxaliplatin on day one, and dexamethasone for four days. Rituximab is given the day before. We don’t know if separating rituximab from chemotherapy is necessary, but if we postulate that the immune system is critical in the mechanism of action of rituximab, then administering rituximab first might be important. The schedule is repeated every 28 days, ideally for eight courses.
This trial is like a Phase I/II study because, in addition to trying to improve response rates, we want to determine the dose of oxaliplatin that can be combined with fludarabine. We began with a low dose that we are now escalating. We’re not using growth factors because neutropenia is not generally pronounced with fludarabine.
The major cumulative toxicity caused by fludarabine involves platelets. After four courses, we sometimes see a delayed drop in platelet counts, which may then stay down and decline with each additional course. Thrombocytopenia is generally in the range of 75,000 to 90,000. It remains at that level for a long time, so sometimes we are only able to give patients, especially elderly patients, six or even four courses.
Duration of rituximab in treating indolent lymphoma
Six cycles has been the magic number for the treatment of aggressive lymphoma, but with indolent lymphomas the response rate is more gradual so we aim for eight cycles. For example, with Burkitt’s lymphoma the majority of patients experience a complete remission with just one course; however, with indolent lymphomas it sometimes takes six courses or more to produce a complete remission. Rituximab has a long half-life, which may explain why some patients take longer to achieve the maximum response.
We have seen patients treated with rituximab, and six months later the lymph nodes were still decreasing in size. I’ve seen one patient whose lymph nodes were still shrinking one year later. In addition, rituximab adheres to tumor cells and is released into circulation as they die.
Curability of Stage IV indolent lymphoma
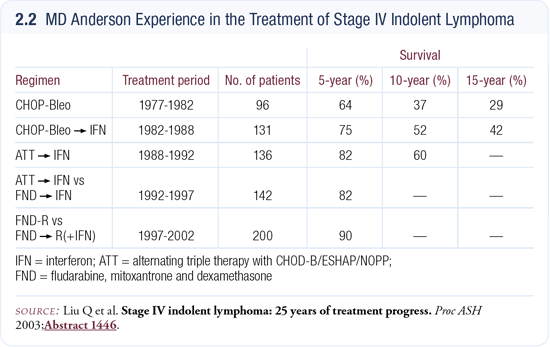
We have reviewed our research experience at MD Anderson in the last 30 years in the treatment of Stage IV indolent lymphomas, motivated by the belief in the oncology community that little progress has been made in the management of this disease. The idea actually came from Stanford. They plotted three decades of their experience in the treatment of indolent lymphoma, and it shows overlapping curves with no improvement at all. Of course, the treatment didn’t change in those 30 years, so I don’t see how outcome could have improved.
We looked at four different studies that we have conducted in the last 30 years and interestingly, a stepwise increase in outcome has occurred every time we opened a new trial (2.2). The survival and failure-free survival both improved, and I’m becoming increasingly convinced that this is not an incurable disorder.
Select publications
| Dr Cabanillas is a Clinical Professor of Medicine at the University of Texas MD Anderson Cancer Center in Houston, Texas and Medical Director of the Auxilio Mutuo Cancer Center in San Juan, Puerto Rico. |
|

