You are here: Home: NHLU 1 2005 : Ian W Flinn, MD, PhD
| Ian W Flinn, MD, PhD |
EDITED COMMENTS |
 ECOG-E4402: Phase III randomized study of rituximab in patients with low tumor burden, indolent non-Hodgkin’s lymphoma ECOG-E4402: Phase III randomized study of rituximab in patients with low tumor burden, indolent non-Hodgkin’s lymphoma
In the ECOG-E4402 trial, patients are treated up front with rituximab in an attempt to delay or prevent administration of chemotherapy. The study has an interesting design. All patients receive four standard doses of weekly rituximab.
Patients are then randomly assigned to either maintenance rituximab, during which a dose is given every three months until progression, or observation, during which they are monitored closely and receive salvage rituximab at the first sign of progression. Treatment continues until the patient is no longer responsive.
Patients are excited about the opportunity to participate in this study. Many patients in my practice have just been diagnosed with low-grade lymphoma. Telling them, “We think you should watch and wait,” is unsatisfactory. Every public service announcement on television — and common sense — tells them that early treatment of cancer is the key to success. With ECOG-E4402 we can tell the patient, “We are not certain that maintenance is going to help you, but it has few side effects and may be beneficial.” That approach is appealing to patients.
Algorithm for transplantation at relapse of indolent lymphoma
For a patient who has had minimal prior therapy and has a matched sibling donor, we would perform a fully ablative transplant. We do a relative T-cell depletion — not a complete T-cell depletion — so it markedly reduces the incidence of graft-versus-host disease. Furthermore, it eliminates many of the infection problems associated with other forms of T-cell depletion. It may also reduce the risk of relapse and the risk of death from the procedure.
For the preparative regimen, we treat with busulfan and cyclophosphamide or cyclophosphamide and total body irradiation, and then the patient receives a graft from the matched sibling (Berdeja 2001). If the patient does not have a matched sibling, we perform an autologous transplant.
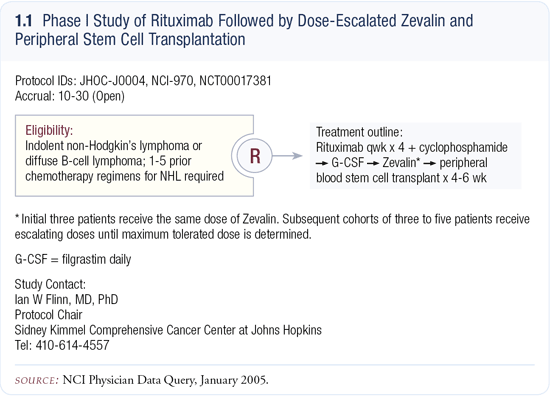
Outside of a clinical trial, we utilize rituximab to purge the stem-cell graft after a high-dose preparatory regimen. Then we administer four doses of rituximab post-transplant. This regimen is offered only to patients in early remissions because patients in later remissions have a very high risk of morbidity and mortality from the procedure. For patients in later remissions, we are developing a new preparative regimen using radioimmunotherapy. This Phase I study employs high doses of Zevalin® (yttrium 90-labeled ibritumomab tiuxetan) and stem cell transplant (1.1).
Nontransplant rituximab/cyclophosphamide regimen
The schema for our nontransplant regimen was based on the ECOG-E2499 trial and previous studies of autologous transplantation in patients with low-grade lymphoma. The regimen consists of four doses of rituximab followed by four doses of cyclophosphamide, using the same dosing regimen we use in patients who receive transplants.
After the patients receive the rituximab and cyclophosphamide, they receive one dose of pegfilgrastim. When their counts return to normal and their platelets recover, they receive two additional doses of rituximab approximately one week apart starting on day 45.
Patients are treated in the outpatient setting and are only admitted if they experience hematologic toxicity. When this occurs, the duration is generally short. This regimen is well tolerated. In fact, I received an email from a patient who was upset that she did not experience side effects and was worried that she was not receiving enough therapy.
Survival benefit with rituximab-containing regimens
Two trials presented at ASH 2004 suggested, for the first time, a survival advantage associated with rituximab-containing chemotherapy regimens. These are preliminary studies and although they do not meet the required level of statistical significance, they suggest a survival advantage. This is big news.
The first trial presented (Herold 2004) is a Phase III study comparing rituximab plus mitoxantrone, chlorambucil and prednisone chemotherapy (R-MCP) to MCP alone. Eligible patients had previously untreated symptomatic Stage III or IV follicular lymphoma, mantle cell lymphoma or lymphoplasmacytic lymphoma. The patients received six cycles of either R-MCP or MCP and were then restaged. Patients who responded received two additional cycles of that respective chemotherapy.
The overall response rate and CR rate were superior with R-MCP compared to MCP; however, the big news was the increase in two-year event-free survival — 83 percent with R-MCP versus 43 percent with MCP alone. Updated results presented at the last ASH meeting suggested an increase in overall survival. Median event-free survival for patients on the rituximab-containing arm had not yet been met.
Although this trial did not use a standard chemotherapy regimen, the principle that combining rituximab with chemotherapy improved survival is important. We need further follow-up from this trial, but this is the first hint of an overall survival advantage with rituximab (Herold 2004).
The second presentation (Van Oers 2004) was a Phase III randomized trial of rituximab in remission induction and maintenance treatment for patients with relapsed or resistant follicular lymphoma. Eligible patients for this two-by-two study had Stage III or Stage IV follicular lymphoma and had previously undergone a maximum of two anthracycline-containing regimens. Patients received R-CHOP or CHOP alone for six cycles. The benefit in CR rate for R-CHOP versus CHOP was highly statistically significant.
The next phase of the trial evaluated maintenance rituximab. The patients evaluable for maintenance were split evenly between the initial groups (CHOP and R-CHOP) and underwent a second randomization to maintenance rituximab or observation. Patients who received maintenance rituximab had a prolonged progression-free survival compared to the control, which is consistent with previous reports.
What was novel about this trial was a trend toward a higher overall survival in patients receiving rituximab maintenance compared with patients randomly assigned to observation. The difference appears to be statistically significant at p = 0.03. However, because it was an early evaluation of a complex study it is not considered statistically significant (Van Oers 2004). This is a large study with enough patients to answer some of the questions that have not been asked in other studies. Ultimately, these findings could change the way physicians practice.
ECOG-E1496: Maintenance rituximab prolongs PFS in advanced indolent NHL
ECOG-E1496 (Hochster 2004) was originally designed to evaluate CVP versus fludarabine plus cyclophosphamide. The fludarabine/cyclophosphamide arm was dropped due to excessive toxicity, and as a result everyone received CVP. Patients were restaged and eligible patients underwent a second randomization to maintenance rituximab or observation. That study demonstrated an increase in progression-free survival but has not shown an overall survival benefit to maintenance; however, it is early, so a difference in overall survival may eventually become evident.
If maintenance rituximab improves progression-free survival, and not overall survival, then I believe the decision of whether or not to administer it is a “dealer’s choice.” On one hand, some patients benefit psychologically by prolonged remission. On the other hand, maintenance therapy leads to time in the infusion chair and is expensive.
Published data suggest that maintenance rituximab will prolong progression-free survival, and emerging data suggest that an overall survival advantage may occur; however, those data are preliminary and are not sufficient for me to use standard maintenance rituximab. When I do use maintenance rituximab, I administer four doses — one every six months for approximately two years.
Select publications
| Dr Flinn is the Director of the Lymphoma Program and Associate Professor of Oncology at the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins in Baltimore, Maryland. |
|

