You are here: Home: NHLU 1 2005 : Oliver W Press, MD, PhD
| Oliver W Press, MD, PhD |
EDITED COMMENTS |
 Management of indolent lymphoma Management of indolent lymphoma
My first consultation with a patient with indolent lymphoma usually involves a long discussion because no routine standard of care exists in the United States.
The treatment of indolent lymphomas is “all over the map” nation-wide. Many options are available, all of which are reasonable and none of which is currently believed to be curative.
Whether survival is prolonged remains controversial — and that is often difficult for the patient to grasp. Patients prefer simple options and one treatment that is clearly best, particularly if it’s a curative treatment. Being presented with an array of options is confusing for most patients.
Off protocol, many patients are followed by observation alone for a few years or treated with a single alkylating agent, such as chlorambucil, or they may receive single-agent rituximab. In the Stanford area they commonly receive R-CVP, which is also a popular regimen in Europe. MD Anderson typically uses a fludarabine-based regimen, such as R-FND.
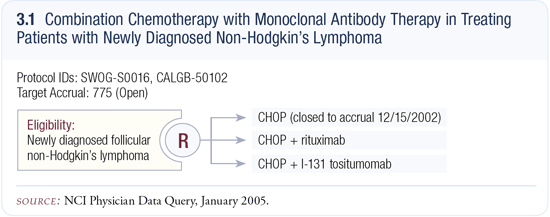
SWOG-S0016: CHOP plus rituximab versus CHOP plus Bexxar®
This trial is evaluating more aggressive therapies for indolent lymphoma (3.1), and was designed to develop a curative treatment or at least one that would prolong survival. It was believed that in order to accomplish that, one might need to combine a chemotherapy regimen with an immunotherapy, such as a monoclonal antibody, because these work by different mechanisms.
Currently, antibodies are generally believed to work best if the tumor burden is relatively small and the antibody doesn’t have to penetrate a long distance to the center of a tumor. CHOP was chosen as the chemotherapy for that trial because it debulks faster and has a higher complete remission rate than many of the other less intense regimens.
Nonprotocol therapy for patients with indolent NHL
Off protocol, I tend to be somewhat more conservative than I would be with patients enrolled in a clinical trial. If a patient is elderly, has relatively small lymph nodes and doesn’t have any symptoms, I will watch and wait until the patient becomes symptomatic or develops bulky disease. Some patients are uncomfortable with watching and waiting but are also afraid of chemotherapy. In those patients, single-agent rituximab is a well-tolerated, mild treatment that often induces a meaningful response.
If patients have substantial bulk of disease and are elderly or have heart disease, I tend to use a combination chemotherapy regimen along with rituximab, such as CVP. In younger patients with rapidly growing bulky disease, I use CHOP plus rituximab.
Role of maintenance rituximab in clinical practice
Rituximab maintenance therapy is currently one of the most controversial issues in the management of indolent lymphomas. Intriguing data have demonstrated that administering either an extended course of rituximab with a dose every two months for four doses (as reported by Ghielmini 2004) or maintenance therapy with four doses every six months for two years (as reported by Hainsworth 2002) may result in a significantly protracted progression-free or event-free survival; however, neither study has shown an overall survival advantage.
If patients are not living longer, and if you’re not even changing the time to rituximab refractoriness — which Hainsworth has shown — then it’s not clear whether maintenance is cost effective. Some oncologists believe that even if you’re not prolonging survival, it is worthwhile to prevent relapses and save patients the mental anguish. Others believe that waiting until relapse to administer rituximab is the preferred approach.
I use rituximab maintenance selectively for patients in whom I’m particularly worried about an early relapse. Perhaps the most common setting where I’ll use maintenance is when a patient comes in after surfing the internet and has strong feelings about wanting rituximab maintenance. I believe the data are strong enough that if someone is inclined to receive it, I administer it.
Sequencing radioimmunotherapy in the treatment algorithm for indolent lymphoma
Off protocol, I believe the best setting for radioimmunotherapy is a patient in their second or third relapse. Like most other treatments, it works best if administered relatively early in the treatment course, and it is a treatment that most patients like because it is a single-dose treatment with few acute side effects. I’ve personally treated many patients who have had durable responses.
On the other hand, the concept of radioactivity is intimidating for some physicians, and the logistic issues have, until now, led oncologists to delay using this therapy until few options remain. Many physicians use it as ninth- or tenth-line therapy, and no treatment will be very effective in those patients.
This is unfortunate, and I’ve been surprised at the slow uptake of radioimmunotherapy. I’ve conducted many of the clinical trials of this approach, and it’s clearly a highly effective treatment — more effective than many of the therapies being utilized more frequently. It is well tolerated and it rarely causes life-threatening toxicity, so I’m almost dumbfounded that it hasn’t captured the imagination of American hematologists and oncologists.
I believe the issues causing the slow uptake involve the logistics of having to coordinate between a medical oncologist, oncologist and a nuclear medicine doctor or radiation oncologist. Reimbursement is also an issue. Additionally, I believe many patients are phobic about radioactivity, even though studies have shown the risks are small.
Active trials with lymphoma vaccines
In single-arm studies, some vaccines appear to prolong remissions. We don’t yet know whether they will cure patients, but I believe the vaccines are one of the most exciting avenues of research being pursued.
Currently, at least three large trials in the United States are evaluating the efficacy of lymphoma vaccines (3.2). The largest — the Genitope trial — was completed within the past year, and the results should be available at the 2005 ASH meeting. In that trial, patients received CVP and were then randomly assigned to receive an idiotype vaccine or not.
A trial by Larry Kwak at MD Anderson is addressing a similar issue but using a doxorubicin-based chemotherapy regimen. The FAV-ID trial utilizes rituximab followed by the vaccine, which is an exciting approach.
Concern exists about whether rituximab, which depletes B-lymphocytes, may blunt the humoral immune response to vaccines, so both the Genitope trial and Larry Kwak’s trial have not allowed rituximab before the vaccine administration. In marked contrast, the FAV-ID trial, or the FAVORAL trial, uses rituximab as the debulking agent before the vaccine is administered.
It will be interesting to see what humoral responses are obtained. Preliminary data from the FAVORAL trial presented at ASH 2004 suggested some patients form antibody responses despite receiving rituximab as induction therapy, but those responses usually are not manifest until the B-lymphocyte depletion resolves (Omer 2004).
Select publications
| Dr Press is a member of The Fred Hutchinson Cancer Research Center, Recipient of the Dr Penny E Petersen Memorial Chair for Lymphoma Research, Professor of Medicine and Biological Structure and Associate Director of the Medical Scientist Training Program at the University of Washington Medical Center in Seattle, Washington. |
|

