You are here: Home: NHLU 3 2005 : Morton Coleman, MD
| Morton Coleman, MD |
EDITED COMMENTS |

PET scans as prognostic tools in large cell lymphoma or Hodgkin’s disease
In patients with large cell lymphoma, we did PET scans around day 18, 19 or 20, prior to the second dose of chemotherapy. In patients with Hodgkin’s disease, we did PET scans just prior to the second cycle of therapy (eg, after two treatments of doxorubicin/bleomycin/vinblastine/dacarbazine [ABVD]) on day 25, 26 or 27 (Kostakoglu 2002). We found that if the PET scan went from positive before therapy to totally negative after one cycle, none of those patients relapsed after nearly two years of follow-up.
In contrast, the overwhelming majority of patients who had a positive PET scan after the first cycle of chemotherapy have relapsed. A few patients who were positive after the first cycle went into complete remission and stayed in complete remission. The majority of these patients had initial standardized uptake value (SUV) scores that were very high and became low, although not back to baseline, after the first cycle of therapy. However, an overwhelming number of patients with high initial, persistent SUV scores relapsed.
We believe the PET scan result after one cycle of therapy is highly predictive of how patients will do in terms of positive and negative predictive value. We found that a PET scan after one cycle of therapy was more predictive than a PET scan at the completion of therapy (Kostakoglu 2002). Others have done PET scans after anywhere from two to four cycles of therapy. Their data are very similar to ours in that if a patient’s PET scan turns negative, the likelihood of relapsing is low. Likewise, if the PET scan remains positive, the likelihood of being cured is not good.
PET scans in patients with transformed lymphomas
The overwhelming majority of patients with low-grade lymphoma tend to have very low SUV scores (ie, two, three, four). If it is suspected that a patient’s disease is transforming and a PET scan is done, one lymph node or organ often has a very high SUV score, which indicates where to obtain a biopsy. We think PET scans are useful in differentiating the transformation from low-grade to high-grade lymphoma. We don’t have enough cases to determine the significance of a negative PET scan after therapy in patients with transformed lymphoma in terms of their overall long-term survival. In fact, transformed lymphoma is far more difficult to treat than de novo aggressive lymphoma.
Nonprotocol therapy for patients with indolent lymphomas
The decision to use rituximab by itself or in combination with chemotherapy often depends on the patient’s presentation. It’s my impression, and data support it, that patients with bulky disease don’t do as well with rituximab alone as they do with combination chemotherapy with rituximab.
Again, a great deal depends on your intent. For a young patient in whom you don’t intend to transplant and would like to achieve a sustained long-term remission, I would suggest using chemotherapy with rituximab.
In terms of which chemotherapy to combine with rituximab, I think you have to be very selective. Chemotherapy selection depends on the patient and what you seek to achieve. I tend toward using fludarabine, mitoxantrone, dexamethasone and rituximab (FNDR). Not much difference exists between FN and CHOP. Some would contend you don’t need to use CHOP and that you can simply use CVP or single-agent therapy with rituximab.
It’s been my tendency to use combination chemotherapy. If I intend to take the patient to a transplant, I may prefer CVP or CHOP, as they are less stem cell toxic. On the other hand, if a patient wants to be engaged in full-time activity, I find that the FND is more convenient, and I don’t have to put in an infusaport. The patients don’t lose their hair, develop neuropathy or experience much nausea with this combination.
However, FND has drawbacks. Fludarabine has been implicated in impacting stem cells. So if future transplantation is being considered, it may not be best to use a combination regimen employing a nucleoside analog. The other disadvantage with FND or any combination with fludarabine is that it is a very potent immunosuppressant. It knocks out T cells to a large degree. This suppression of T cells translates into the potential for opportunistic infections.
Maintenance therapy with rituximab
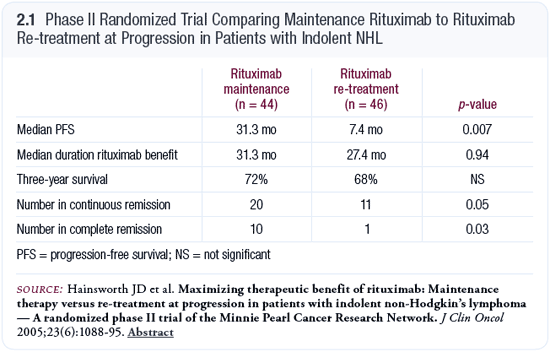
Maintenance rituximab remains a highly controversial subject. The most telling study to date is the one by Dr Hainsworth, although it was certainly underpowered. This study evaluated maintenance rituximab versus re-treatment upon relapse. He found that the total amount of time a patient would derive benefit from rituximab was the same whether they received maintenance rituximab or were re-treated upon relapse. Nevertheless, patients who received maintenance rituximab tended to stay in remission longer (Hainsworth 2005; [2.1]).
Besides being more expensive, when maintenance rituximab is used, almost all of the patients become hypogammaglobulinemic. A small subset of those patients develops chronic sinusitis, which does not respond to antibiotics or any other type of symptomatic treatment. These patients ultimately require treatment with gamma globulins in much higher doses than we commonly use. I don’t view rituximab as an innocuous therapy.
I bring up the issue of maintenance rituximab with my patients, and sometimes I believe maintenance rituximab is necessary. In situations in which I’m worried that relapse will carry additional problems, I maintain the patients on rituximab. For example, Waldenstrom’s macroglobulinemia can often be associated with neuropathy. We don’t want patients to relapse and have the neuropathy return. Therefore, in patients with Waldenstrom’s macroglobulinemia, I use maintenance rituximab. It’s important to obviate any kind of complication that may be a result of the malignancy.
Select publications
| Dr Coleman is a Clinical Professor of Medicine at Weill Medical College of Cornell University and Director of the Center for Lymphoma and Myeloma, New York Presbyterian Hospital, New York, New York. |
|

