
 |
||||||||
| |

| Tracks 1-18 | ||||||||||||||||||||||||||||||||||||||
|
Select Excerpts from the Interview
![]() Track 2
Track 2
![]() DR LOVE: What are some of the questions being studied right now in
diffuse large B-cell lymphoma research?
DR LOVE: What are some of the questions being studied right now in
diffuse large B-cell lymphoma research?
![]() DR LEONARD: Number one, will CHOP remain the standard therapy? We’re
conducting a randomized trial of R-CHOP versus R-EPOCH (2.1) based on
Wyndham Wilson’s data and some Phase II data that the CALGB generated
(Gutierrez 2000; Wilson 2001).
DR LEONARD: Number one, will CHOP remain the standard therapy? We’re
conducting a randomized trial of R-CHOP versus R-EPOCH (2.1) based on
Wyndham Wilson’s data and some Phase II data that the CALGB generated
(Gutierrez 2000; Wilson 2001).
The GELA is evaluating R-CHOP-21 versus R-CHOP-14. Their data supporting the 14-day schedule were generated primarily in the prerituximab era, so whether or not the 14-day, dose-dense treatment will be useful in lymphoma in the rituximab era remains to be seen. Some people in practice are using R-CHOP-14, but most are using R-CHOP-21, and I believe that’s an important question.
Finally, evaluating new biologic agents and antibodies is another issue. Bevacizumab is being considered in lymphoma. And we have enzastaurine, a protein kinase-C inhibitor. Those are questions that people are considering, and the challenge is that many of these agents may have only marginal, single-agent activity in large cell lymphoma.
Do you go forward and conduct a large trial with hundreds of patients without much single-agent activity because you believe the agents will be chemosensitizers?

![]() Track 3
Track 3
![]() DR LOVE: What is known about bevacizumab in lymphoma?
DR LOVE: What is known about bevacizumab in lymphoma?
![]() DR LEONARD: We’re planning a Phase II trial in mantle-cell lymphoma
with CHOP, rituximab and bevacizumab as up-front therapy. We have a lot
of interest in angiogenesis and lymphomas. The data with bevacizumab in
lymphoma have been generated primarily from two studies.
DR LEONARD: We’re planning a Phase II trial in mantle-cell lymphoma
with CHOP, rituximab and bevacizumab as up-front therapy. We have a lot
of interest in angiogenesis and lymphomas. The data with bevacizumab in
lymphoma have been generated primarily from two studies.
One was from the Southwest Oncology Group and was presented previously at ASCO (Stopeck 2005). The other was a small pilot trial from ECOG that evaluated R-CHOP with bevacizumab (Ganjoo 2006). They demonstrated activity, but obviously, learning what the bevacizumab added to the R-CHOP would require a randomized trial.
![]() Track 11
Track 11
![]() DR LOVE: What is your clinical approach to patients with mantle-cell
lymphoma?
DR LOVE: What is your clinical approach to patients with mantle-cell
lymphoma?
![]() DR LEONARD: I observe patients with mantle-cell disease for a while if they’re
asymptomatic. Mantle cell is almost like a FLIPI high-risk follicular lymphoma
in that the patients have incurable disease, but in some cases it can be indolent
and they can go off therapy. We’ve been studying R-CHOP with bortezomib.
DR LEONARD: I observe patients with mantle-cell disease for a while if they’re
asymptomatic. Mantle cell is almost like a FLIPI high-risk follicular lymphoma
in that the patients have incurable disease, but in some cases it can be indolent
and they can go off therapy. We’ve been studying R-CHOP with bortezomib.
You must decide whether a patient is a transplant candidate and whether a more aggressive approach will be part of your treatment plan. If not, with a 75- or 80-year-old patient with mantle-cell disease you can obtain reasonably good mileage out of an R-CHOP-type regimen.
However, these are patients for whom I believe bortezomib will also play an important role because with it, you can achieve good disease control without the toxicity of the more aggressive regimens.
![]() DR LOVE: What are your thoughts on Brad Kahl’s modified R-hyper-CVAD
regimen?
DR LOVE: What are your thoughts on Brad Kahl’s modified R-hyper-CVAD
regimen?
![]() DR LEONARD: R-hyper-CVAD is a challenging regimen for patients because
of the toxicities. The data have been impressive, but we have to be mindful of
patient selection issues.
DR LEONARD: R-hyper-CVAD is a challenging regimen for patients because
of the toxicities. The data have been impressive, but we have to be mindful of
patient selection issues.
The idea of being in the hospital for six months to receive hyper-CVAD for the possibility of a progression-free survival benefit or an overall survival benefit is difficult to weigh.
The idea with the modified regimen has been to reduce the toxicity and cut out the methotrexate/ARA-C, which is the more toxic portion of the regimen. I’ve seen reports of two- to three-year progression-free survival, which certainly appears reasonable.
![]() Track 13
Track 13
![]() DR LOVE: How do you approach the off-protocol clinical management of
the patient with indolent lymphoma?
DR LOVE: How do you approach the off-protocol clinical management of
the patient with indolent lymphoma?
![]() DR LEONARD: I feel strongly about observing these patients for a while. I try
to watch for three to four months because that provides the clinician a sense of
what to expect out of the disease and allows the patient to become comfortable
with the disease. It’s counterintuitive that you can be diagnosed with
cancer and your doctors want to simply watch and wait, and certainly clinicians
know the challenges of spending an hour explaining to a patient why he
or she does not need treatment.
DR LEONARD: I feel strongly about observing these patients for a while. I try
to watch for three to four months because that provides the clinician a sense of
what to expect out of the disease and allows the patient to become comfortable
with the disease. It’s counterintuitive that you can be diagnosed with
cancer and your doctors want to simply watch and wait, and certainly clinicians
know the challenges of spending an hour explaining to a patient why he
or she does not need treatment.
When I treat patients, I tend to use concurrent R-chemotherapy regimens. I am more in the R-CVP camp than anything. I tend to use the anthracycline later in case the disease transforms. Certainly a number of people use fludarabine- based regimens, but I use those more sparingly.
![]() Track 14
Track 14
![]() DR LOVE: What is currently happening in terms of clinical research of
radioimmunotherapy (RIT) for lymphoma?
DR LOVE: What is currently happening in terms of clinical research of
radioimmunotherapy (RIT) for lymphoma?
![]() DR LEONARD: Most of the data coming out now have been from Phase II
trials evaluating chemotherapy combinations — largely in indolent lymphoma,
some in aggressive lymphoma — with R-chemotherapy followed by RIT.
These studies have all suggested that you can use these combinations. They
have acceptable toxicity and high response rates, but they’re all incorporating
chemotherapy regimens that have high response rates.
DR LEONARD: Most of the data coming out now have been from Phase II
trials evaluating chemotherapy combinations — largely in indolent lymphoma,
some in aggressive lymphoma — with R-chemotherapy followed by RIT.
These studies have all suggested that you can use these combinations. They
have acceptable toxicity and high response rates, but they’re all incorporating
chemotherapy regimens that have high response rates.
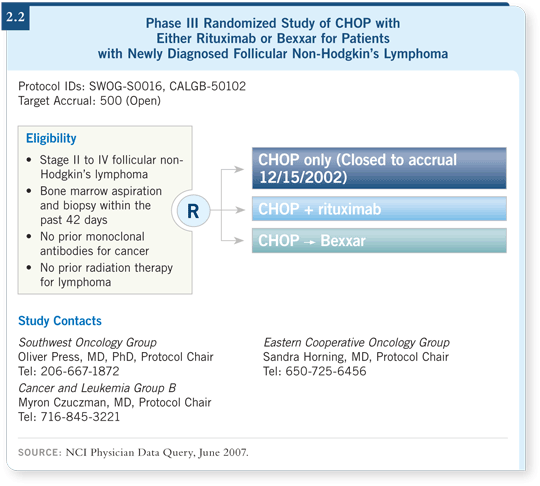
The primary trial that will answer the question of RIT consolidation after chemotherapy will be the SWOG-S0016 trial (2.2) of R-CHOP versus CHOP followed by Bexxar as initial treatment for follicular lymphoma.
In this study, R-CHOP is not followed by RIT, and if it were designed today, it probably would be designed to include that arm. I believe if it’s a positive trial, some people may extrapolate to that. We have relatively few data on RIT after rituximab-containing regimens. Ollie Press has presented preclinical data that provide some insight into this combination. The concern is that if you have rituximab circulating from the R-CHOP at the time you administer the RIT, it will block out the radiolabeled antibody.
He presented some data suggesting that might be the case. One might argue that if you were going to use that strategy — let’s say if you were administering six cycles of R-CHOP — maybe you’d administer R-CHOP the first four and then CHOP alone, so you’d clear the rituximab before coming in with RIT.
In clinical practice, RIT is an underused option for many patients, in part because people are just now becoming familiar with it. However, data suggest that it can be helpful for patients with rituximab-refractory disease and for patients with chemotherapy-refractory disease with indolent and transformed lymphomas. I tend to use it for people who have responsive disease but shorter remissions.
| Table of Contents | Top of Page |