You are here: Home: NHLU 4 2005 : Bruce D Cheson, MD
| Bruce D Cheson, MD |
EDITED COMMENTS |
 Treatment advancements in NHL Treatment advancements in NHL
The most revolutionary occurrence in the treatment of patients with NHL has been the availability of active monoclonal antibodies, particularly rituximab. This has provided a wonderful building block on which to develop newer and more effective regimens, both in combination with chemotherapy and other biological agents. One of the most important advances in the last year or two has been the recognition that rituximab adds to the activity and efficacy of chemotherapy.
The Groupe d’Etude des Lymphomes de l’Adulte compared CHOP with or without rituximab in patients with diffuse large B-cell lymphomas and demonstrated a survival advantage (Coiffier 2002). Several other groups have confirmed these results, and R-CHOP has replaced standard CHOP as a more effective therapeutic option for patients with diffuse large B-cell lymphomas. In patients with indolent lymphomas, rituximab also adds to the efficacy of chemotherapy. Compared with chemotherapy alone, rituximab plus chemotherapy enhances the response rate and time to progression. Although a survival benefit has not been demonstrated, it may become apparent with longer follow-up (Forstpointner 2004; Hiddemann 2004; Marcus 2005).
Rituximab Extended Schedule Or Re-Treatment (RESORT) trial
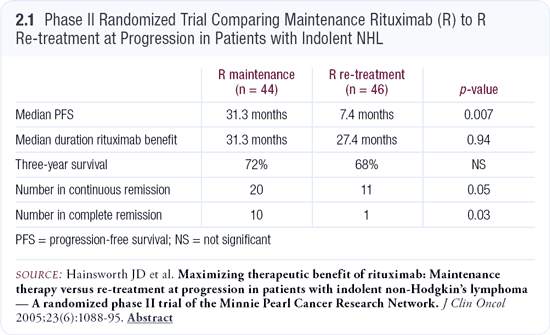
In the RESORT trial, patients with indolent lymphomas receive the standard four weekly infusions of rituximab. Then they are randomly assigned to: (1) observation until disease progression, at which time they receive rituximab re-treatment or (2) rituximab every three months until their disease progresses (1.1). It’s somewhat similar to a study conducted by Hainsworth et al, in which they administered rituximab for four infusions followed by either maintenance for two years (eg, four infusions every six months) or no further treatment until relapse (Hainsworth 2005). In that study, which involved patients with relapsed or refractory disease, there was a marked difference in time to progression with maintenance rituximab. However, the time at which patients needed some therapy other than rituximab was virtually identical: 31 months in the maintenance arm and 27 months in the re-treatment arm (Hainsworth 2005; [2.1]).
To date, no regimen prolonging time to progression has impacted on overall survival. However, those results have been primarily with the older forms of treatment, and hopefully, with antibody therapy, we’ll find we not only prolong time to progression but also survival. Obviously, concerns exist about keeping patients on a monoclonal antibody indefinitely. One concern is the expense. The other concerns include: Are there risks of chronic B-cell depletion? Will patients be at risk several years down the line for bacterial or other forms of infections? Will they develop some form of resistance to monoclonal antibody therapy?
Clinical use of maintenance rituximab
Some recent abstracts suggest that maintenance rituximab may be a beneficial approach (Hiddemann 2005; Habermann 2004; Van Oers 2004), but we haven’t seen a full manuscript we can critically evaluate to demonstrate that this is the way to go. So we use maintenance rituximab, currently, in the context of a clinical trial. We do present maintenance rituximab to patients as an option, and I won’t say we never use it. When we discuss maintenance rituximab with patients, I usually present a balanced view of the pros and cons. Most of the time, they decide to receive it at the time of recurrence rather than continuously. The advantage we pose to them is that maintenance rituximab will prolong the time to disease progression, but we don’t know whether it will prolong survival.
Potential survival advantage associated with rituximab
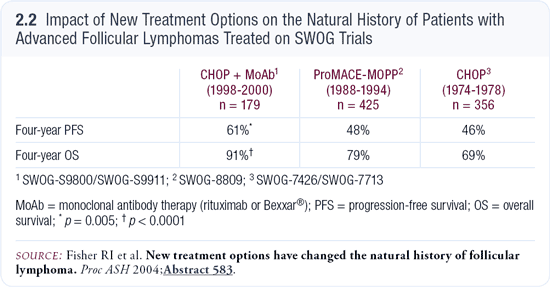
Two recent studies go against our former concept that we can’t prolong the survival of patients with follicular lymphoma. They demonstrate that recent regimens containing monoclonal antibodies, particularly rituximab, appear to enhance survival when compared with comparable historical controls from clinical trials. One trial is in press in JCO. The other study was conducted by the Southwest Oncology Group (Fisher 2004). Dr Fisher conducted a retrospective analysis of a large number of patients treated with CHOP, a more aggressive chemotherapy regimen (ProMACE-MOPP) and CHOP followed by monoclonal antibody therapy. Although the duration of follow-up was much shorter with the antibody-containing regimen, both the time to progression and the survival curves were significantly in favor of the antibody-based therapy (Fisher 2004; [2.2]). Whether these differences will remain with prolonged follow-up is yet to be seen.
Select publications
| Dr Cheson is the Head of Hematology and Director of Hematology Research at Georgetown University Hospital’s Lombardi Comprehensive Cancer Center in Washington, DC. |
|

