You are here: Home: NHLU 4 2005 : Myron S Czuczman, MD
| Myron S Czuczman, MD |
EDITED COMMENTS |
 SWOG trial S0016 in patients with newly diagnosed follicular NHL SWOG trial S0016 in patients with newly diagnosed follicular NHL
SWOG-S0016 was originally a three-arm study comparing CHOP alone, CHOP plus rituximab (R-CHOP) and CHOP times six followed by a dose of Bexxar. Early on, the trial was not accruing well, largely because patients said, “I don’t want to be on CHOP alone.”
Right now, it’s a two-arm study comparing R-CHOP to CHOP followed by Bexxar (3.1). Hopefully, this trial will accrue enough patients. It’s a large cooperative group study, and it’ll be interesting to see which of these approaches may be better to determine their long-term toxicities.
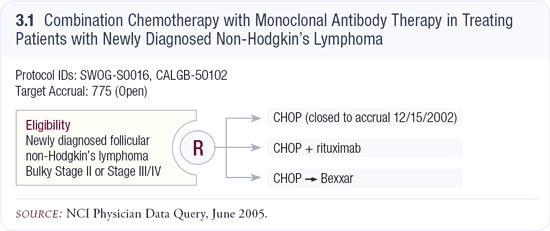
Background for SWOG-S0016
CHOP plus rituximab
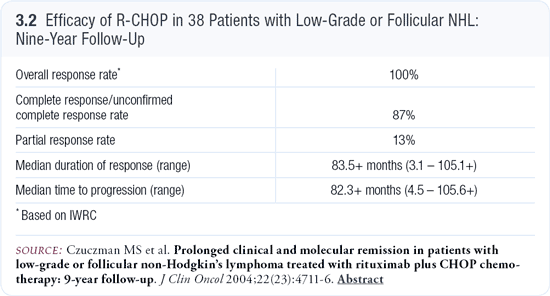
We just updated the nine-year experience of a multicenter trial evaluating the combination of rituximab and CHOP, and the data are intriguing (Czuczman 2004; [3.2]). Patients with either untreated or previously treated (up to four different treatment regimens) follicular or low-grade lymphoma were eligible. Patients could not have bulky disease (eg, >10-centimeter masses). They received six cycles of CHOP along with six infusions of rituximab (Czuczman 1999). Thirty-eight of the 40 patients who enrolled were treated. The two who were not treated went off study before therapy was initiated (Czuczman 2004).
We had a 100 percent overall response rate. In the update of the nine-year followup, we applied the International Workshop Response Criteria (IWRC) for NHL published by Cheson et al (Cheson 1999). When we applied those criteria and compared to our more rigid initial criteria, we noted an 87 percent complete response/unconfirmed complete response (CR/CRu) rate and a 13 percent partial response (PR) rate. We have reached a median time to progression of close to seven years (Czuczman 2004; [3.2]). Therefore, at seven years, half of the patients have relapsed, and the other half are still going strong.
All 16 patients who are continuously in complete remission had an initial CR. All five patients with a PR according to the IWRC had relapsed by 2.5 years (Czuczman 2004). I think that may be a very important point. From the data I’ve seen with Zevalin® and Bexxar, this database and others published with chemotherapy, the patients who achieve a CR have the most durable and meaningful remissions, sometimes lasting five-plus years.
I have patients whom I treated on the R-CHOP study now going out 10 years. Being a clinician, I would not have predicted they would stay in remission that long. We also saw molecular remissions in our trial (Czuczman 2004). In a number of publications and in this database, it appears that patients who achieve a molecular CR do better than those who achieve only a clinical CR.
CHOP followed by Bexxar
The basis for CHOP followed by Bexxar was a trial conducted by Dr Press in Seattle. Over 100 patients were treated with six cycles of CHOP followed by Bexxar. They had an excellent overall CR rate and durable remissions (Press 2003). The follow-up in that trial is not as long as the follow-up in the trial of R-CHOP we’ve published. When you look at the same time frames, however, they appear to be comparable.
Since then, Mark Kaminski has published a trial of Bexxar alone as up-front therapy. Patients who were enrolled in that trial needed to have less than 25 percent marrow involvement, and the majority did not have bulky disease. In general, they were probably a good prognostic group of patients, and they did very well (Kaminski 2005). I think that trial involved a select group of patients who might have done well with rituximab alone. Data from the French suggest that around 25 percent of “good-risk” patients, at five years, are still in CR.
Phase II trial of rituximab plus fludarabine in patients with low-grade or follicular NHL
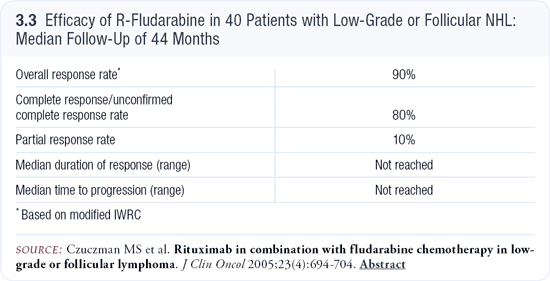
We’ve published, in JCO, the four-year follow-up of a trial with rituximab plus fludarabine (R-fludarabine). Six cycles of standard fludarabine and seven infusions of rituximab were administered. We had a 90 percent overall response rate and an 80 percent CR rate. At almost four years, we had not reached the median time to progression (3.3). We still had a little over 60 percent of the patients in remission. Toxicity was very acceptable, and nonhematologic toxicity was minimal (Czuczman 2005). Patients could work full time and were active. The treatment did not cramp their lifestyles.
In the first 10 patients, we saw too much hematologic toxicity, and we adjusted the protocol. We discontinued Bactrim® (trimethoprim/sulfamethoxazole) prophylaxis, because we weren’t using steroids with fludarabine, as in the FND regimen, which leads to a higher risk of developing pneumocystis carinii pneumonia. If the patients experienced prolonged cytopenias, we reduced the dose of fludarabine from five to three days. We also limited the amount of G-CSF, because we would sometimes have worse neutropenia if filgrastim were administered soon after fludarabine was completed (Czuczman 2005).
Two patients had to be taken off of the study due to disease progression, and those two patients had transformed lymphomas. So I think R-CHOP is probably better for patients with a more aggressive presentation. In addition, the patients required acyclovir prophylaxis, because about 15 percent developed either primary or secondary herpes infections. With the use of acyclovir, we saw no other herpes infections, and no other opportunistic infections occurred (Czuczman 2005).
Comparing R-CHOP and R-CVP as initial therapy in patients with follicular lymphoma
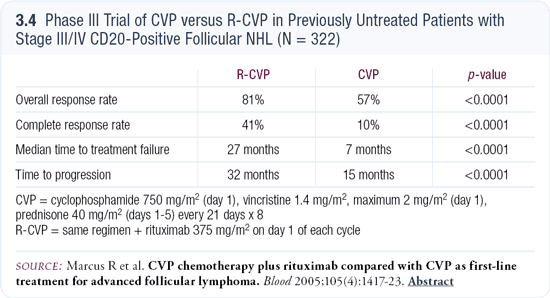
I don’t use R-CVP. I trained at Memorial Sloan-Kettering, and R-CHOP, at that time, was not a bad thing. Anthracyclines were not evil, but many don’t like to use them. If you look at historical data to compare CVP to CHOP, you can have a quicker response with the addition of doxorubicin, but you don’t necessarily change overall survival.
When I look at the data recently published by Marcus from the randomized trial comparing CVP to R-CVP, what strikes me the most — although R-CVP beats the CVP — is that the median time to treatment failure was 27 months for R-CVP (Marcus 2005; [3.4]). I’m seeing patients whom I have treated with R-CHOP going out seven, eight-plus years; therefore, less than 2.5 years is not very satisfying. In my own interpretation of the data, R-CHOP is providing better results.
Preclinical work and in vitro studies demonstrate that doxorubicin and rituximab have synergy. In my mind, I’m not just treating the patient with CHOP. I’m adding rituximab, which is synergistic with doxorubicin. Hence, I’m providing a better chance of having a quality remission and taking advantage of the synergy. By using CHOP or R-CHOP early on, I have not seen, in my own series of patients, a very high rate of transformation in patients with low-grade lymphomas. This has to be studied prospectively, but I think it makes a very good question.
Maintenance rituximab versus re-treatment with rituximab
In some patients who have minimal treatment options, I do consider utilizing maintenance rituximab. When I look at the Hainsworth data, it is clear that patients had the same duration of benefit with rituximab whether it was administered as four doses every six months for two years or at the time of progression (Hainsworth 2005). If you continue administering rituximab without a good reason, there may be a theoretical increased risk of developing biological resistance in the primary tumor cells, which we’re studying in the laboratory.
If I have a patient who appears to have early relapse following an autologous transplant (eg, the nodes are slowly progressing, but they’re too small to obtain a biopsy), I have no problem using rituximab weekly times four. In a number of cases, I have seen their tumors regress. I use a common-sense approach. If the patient’s disease is progressing, the patient is post an autologous transplant, the patient is elderly or the patient did not handle chemotherapy well in the past, I don’t have a problem using rituximab at the time of re-treatment.
Mantle-cell lymphoma is another condition in which you have older patients who may not be able to tolerate an aggressive hyper-CVAD regimen. If patients are treated with R-CHOP and are not candidates for transplantation, then maybe using set doses of rituximab on a regular basis is reasonable.
In CALGB, one of the trials we’re discussing takes patients in first CR to an autologous stem cell transplant, and we’re adding some maintenance type of treatment. We’re discussing bortezomib alone or in combination with rituximab, although rituximab may be used in combination with other agents.
Select publications
| Dr Czuczman is the Head of Lymphoma/Myeloma Service at Roswell Park Cancer Institute and Associate Professor of Medicine in the School of Medicine and Biomedical Sciences at the State University of New York at Buffalo, New York. |
|

