You are here: Home: NHLU 4 2005 : Editor's Note
 |
|
Is overall survival the only important endpoint in Phase III
randomized clinical trials? |
In the early 1980s, a number of adjuvant clinical trials in early breast cancer demonstrated a disease-free survival advantage for tamoxifen. However, at that time, the concept of targeted adjuvant therapy was still embryonic, and most oncologists did not prescribe TAM in this setting believing that “cytostatic” therapy might temporarily delay tumor growth but would not impact overall survival (OS).
It was not until the 1985 NIH Consensus Conference on Early Breast Cancer that Oxford statistician Richard Peto’s first international meta-analysis proved that the lack of OS benefit in individual trials of tamoxifen was the result of too few events (deaths), rather than a lack of efficacy. Peto’s clear-cut demonstration of an OS impact instantly changed the standard of care and led a generation of women worldwide to take TAM in the early setting. However, even prior to Peto’s presentation, a number of clinical investigators began to question whether a disease-free survival (DFS) benefit alone was sufficient enough to justify the use of a treatment with relatively few side effects or toxicities. The OS requirement of the “pre-Peto” era stemmed in large part from the significant toxicity profile of cytotoxics observed in prior adjuvant studies. It is interesting to speculate whether the rationale of using TAM for a DFS advantage would have ever gained acceptance if Peto and his colleagues never addressed the issue of breast cancer.
Twenty years after Peto’s bombshell, we can reflect on several other oncologic situations in which disease-free survival benefits have been accepted to justify new standards of care. At the 2001 San Antonio Breast Cancer Symposium, the first results of the massive ATAC trial demonstrated a disease-free survival benefit for the use of the aromatase inhibitor anastrozole over tamoxifen in postmenopausal patients. More than four years later, this study and several other similar trials have still not revealed an OS benefit for AIs over tamoxifen; yet, these agents are now the most commonly utilized endocrine intervention in early breast cancer. Another example of DFS justifying a therapy occurred after the 2003 ASCO meeting, in which Aimery de Gramont and colleagues demonstrated that FOLFOX conferred a three-year DFS advantage over 5-FU/leucovorin as adjuvant therapy for Stage III colorectal cancer. Part of the acceptance of these data as the basis to change practice was the expectation that this DFS benefit would eventually translate into a five-year OS benefit. However, a follow-up MOSAIC data set presented at the recent 2005 ASCO meeting continues to reveal no OS advantage. FOLFOX, however, remains the accepted standard of care, in spite of the considerable increase in toxicity associated with this treatment. In NHL, the much-discussed and controversial topic of rituximab maintenance seems to fit this model well. As in virtually every other issue of this audio series, the three interviewees featured on the enclosed program identify the role of rituximab maintenance therapy as among the most common questions asked of them by medical oncologists.
There is considerable heterogeneity in the approach taken by lymphoma specialists to this question, but most believe — mainly based on John Hainsworth’s study of R maintenance in patients treated initially with R monotherapy for relapsed indolent lymphoma — that continuation of R will initially delay tumor progression. There is also general agreement that other than financial cost and inconvenience, there are few known risks of R maintenance. The controversy stems from two questions:
1. Would the same long-term tumor control be observed without R maintenance, and R was not utilized until progression?
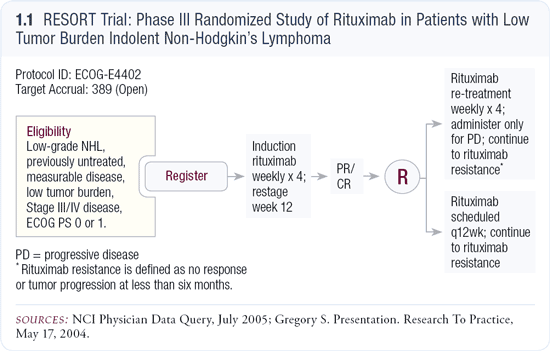
As discussed on a previous issue of this series by principal investigator Brad Kahl, ECOG’s RESORT trial is attempting to address this critical question (1.1). Of particular interest, in RESORT, R maintenance is administered indefinitely, whereas Hainsworth utilized only two years of therapy — a common nonprotocol approach currently used. A key endpoint in RESORT is the time to first chemotherapy, which obviously has important quality-of-life implications. If R maintenance does not improve OS but leads to a significant delay in the first use of chemotherapy, this may be viewed as a positive benefit-risk ratio.
2. Will overall survival be improved with R maintenance?
This research issue is clearly the major impetus to conduct any study of an early versus delayed systemic intervention in oncology. For aggressive tumors such as diffuse large B-cell lymphoma, overall survival is a rational endpoint that can be assessed fairly quickly. In this issue of NHL Update, James Armitage comments on a population-based study from British Columbia that demonstrated fewer deaths from diffuse large B-cell lymphoma within the 18 months after the Canadian government approved rituximab.
However, the prolonged natural history of indolent lymphomas means that any trial with a primary endpoint of overall survival may be impractical and likely to deliver results at such a delayed time point that newer and more effective forms of treatment will already be available. It is also possible that in some way, R maintenance may result in inferior long-term tumor control and OS, but few if any clinical investigators have raised that as a serious concern. The primacy of OS as a trial endpoint triggering changes in practice patterns is logical when the intervention results in significant short- and long-term toxicity, but what about therapies like rituximab that result in minimal adverse effects?
For the foreseeable future, it seems that oncologists must develop a clinical strategy that takes into account these uncertainties. This is no small task, particularly in light of the recent emergence of a number of new effective biologic treatment strategies that have substantially increased the overall financial burden for cancer placed on society. Even more importantly, oncologists must decide whether to proactively discuss issues such as R maintenance with patients, even if only to say, “This is an option that some oncologists use in this situation, but I am not recommending this to you because...”
In some ways, the R maintenance question reminds me of the current debates about tamoxifen versus an AI as up-front adjuvant therapy. Everyone agrees that AIs result in fewer relapses in the short term, but some have argued that better long-term results may occur if tamoxifen is administered initially for two to three years followed by the AI. The only major, large, prospective randomized trial (BIG FEMTA) addressing this question will not have results for years.
Putting aside the issue of the differential side effects of these therapies, oncologists have understandably balked at recommending that patients accept initial treatment with a greater risk of relapse (tamoxifen), hoping and expecting that in the long term, fewer relapses will occur. Similarly, by foregoing R maintenance, patients with NHL are being asked, in essence, to go with a treatment strategy that in the short term will result in a greater likelihood of tumor progression, with the expectation/hope that in the long term, the outcome will probably be the same. We and others have conducted a number of patient surveys in breast, prostate and colorectal cancer to determine how patients balance riskbenefit considerations to make treatment decisions. It would be fascinating to ask patients with NHL how they see this trade-off when it is explained in this manner.
— Neil Love, MD
NLove@ResearchToPractice.net
|

